

 |
 |
Tenthredinidae (Hymenoptera, Symphyta) are the largest family of plant-feeding Hymenoptera. Larvae usually feed externally on plants, although there are quite a few endophytic species (for instance, all Fenusini and Heterarthrini are typical miners.) The subfamily Nematinae, which constitutes one of the most distinct and advanced groups in the family, includes many species with concealed feeding habits.
Nematinae are strikingly different from the rest of Tenthredinidae in reduction of the number of prolegs, which present on abdominal segments 2-7 and 10 rather than 2-8 and 10 (although similar reduction is found in some Fenusini). It is only in the nematine larvae that we find median ventral eversible glands. In the free-living species, these protruding odorous appendages are believed to play a repellent role. Glands of this kind in the gall-making Pontania as well as other endophytic nematine groups have been considered as evidence of the origin of these groups from some free-living ancestors (Smith 1970). However, the fact that endophytic groups possess ventral glands can hardly be considered proof of their descent from free-living ancestors, since it is hardly possible that the current function of defense had been their original function. On the contrary, it is far more possible that the free-living forms originated from those with a concealed feeding habit. The purpose of this article is to justify this assumption.
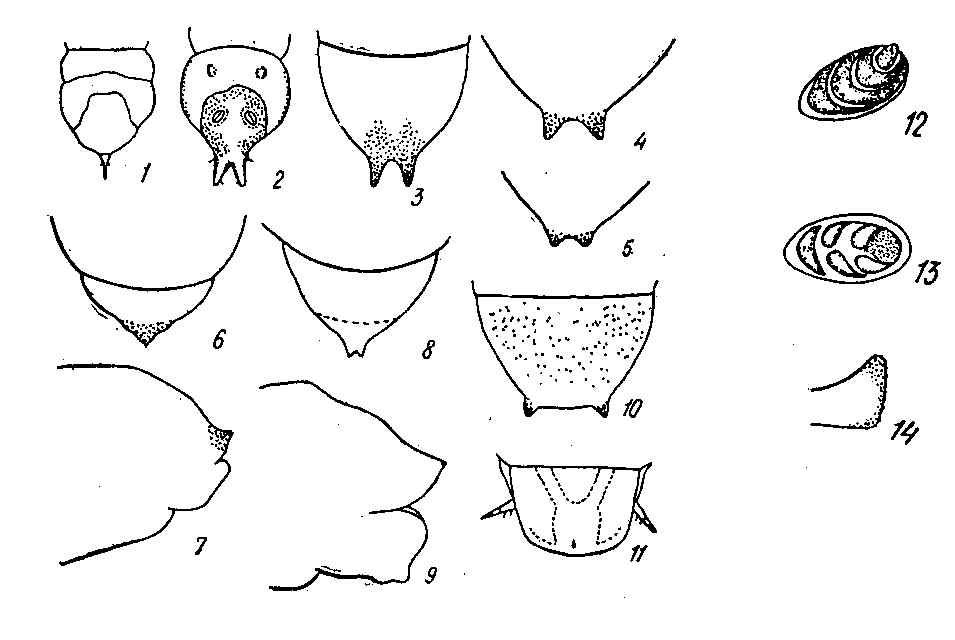
The reduction of antennal segments is an important diagnostic character of nematine larvae and may serve well as evidence in favor of this hypothesis. In the entire family, this kind of reduction is typical only in highly specialized miners, such as Fenusini, Heterarthrus, as well as Nematinae, including their concealed as well as free-living members. This fact can be anything but an accidental phenomenon. The rest of the Tenthredinidae larvae have very distinct conical five-segmented antennae. Within the Nematinae, only Hemichroa larvae are known to have five-segmented antennae (Lorenz & Kraus 1957). The majority of Nematinae have four- and even three-segmented short antennae that hardly or not at all project above the surface of the head capsule (Fig. 12, 13).
Of all the Nematinae, it is only in a few free-living species that the most developed antennae are found. As for the endophytic Nematinae, fully developed antennae are found only in the most primitive group, Hoplocampa. One can attribute the distinct reduction of the antennae to the advanced evolutionary status of the group as well as to the endophytic habit of larvae. The reduction of the antennae is particularly typical for such highly advanced and, at the same time, endophytic groups as Pontania and Euura. On the other hand, similar, completely flat antennae are also found in some larvae with free-living habits in advanced as well as primitive Nematinae (Fig. 13). This could be attributed to the fact that the endophytic habit was common in ancestors of the more recent groups.
Another distinctive feature of many nematine larvae is the presence of a pair of 'caudal protuberances', the so-called cerci or, to be more precise, pseudocerci. Although they constitute protrusions at the posterior margin of the last tergite, they are not homologous to the cerci of adults, nor to ones of other insects (Middleton 1921). Both the shape and position of the pseudocerci in Nematinae are greatly variable. In the free-living Hemichroa larvae, the entire margin of the anal tergite is armed with multiple short denticles; other Nematinae may have only a single pair of pseudocerci.
Pseudocerci may be positioned laterally, as in Phyllocolpa (Fig. 10) and some Pontania with concealed feeding habits as well as in free-living Croesus, Nematus, and many Amauronematus. In some free-living species, the lateral pseudocerci are fully developed and swollen at their apices; in others, they may be nearly completely reduced.
Until recently, medially positioned pseudocerci have been known exclusively in endophytic groups, such as Caulocampus acericaulis (MacGillivray) (a miner in maple leaf petioles) as well as some gall-making Pontania. In both groups, the last tergite is attenuated into the medial caudal protrusion bearing small medial pseudocerci at its apex (Fig. 8, 9); in Caulocampus, the attenuation is chitinized (Yuasa 1922).
Typical medial pseudocerci recently have been found among free-living nematine larvae belonging to the subgenus Nematus (Paranematus) (Vikberg 1972, Zinovjev 1978). In Paranematus the development of the caudal protrusions may be highly variable in different species as well as different specimens of the same species. The pseudocerci tend to become shorter with age of larvae; in the last instars pseudocerci are shortened or even reduced to inconspicuous tubercles in some specimens (Fig. 3, 4, 5).
Caudal appendages are known in many different insects; however, they are most characteristic of those with concealed feeding habits. These appendages may be used for excavation (similarly to those of the Ipidae), or be organs of support and locomotion, or else sensors. Sensor appendages are often found laterally, as, for example, those of Pamphiliidae and Megalodontidae larvae, which live in webbed nests or leaf curls. In case the support function dominates, the medial location appears to be most efficient. The extreme example is probably the single supra-anal thorn (or cornus), which is found in Siricidae, Xiphydriidae, and Cephidae larvae (Fig. 1). Appendages that are considered to be organs of support are known in many other insects: cremasters in mobile Noctuidae pupae, urogomphi in Elateridae larvae, similar appendages in Tenebrionidae and Xylophagidae (Fig. 2), and others (Gilyarov 1949, Gilyarov 1964, Krivosheina 1969, and others). In Tenthredinidae, some species of Fenusa appear to have an analogous organ, a single denticle (Fig. 6, 7). Apparently, its development may be attributed to the mining habit of Fenusini as well as to the reduction of the last pair of prolegs.
The medial pseudocerci of Caulocampus and Pontania, as mentioned by Yuasa (1922), most probably have the same function as the supra-anal thorn of Siricidae. There is striking similarity between the pseudocerci (the lateral as well as medial) of nematine larvae and support appendages of a vast variety of insects with concealed feeding habits (Fig. 3-10). This resemblance may be interpreted as evidence of correlation between the development of this kind of organs and the concealed feeding habit. The wide occurrence of pseudocerci among free-feeding larvae might point to their origin from endophytic ancestors.
It is worth mentioning that the dorsal swellings in the larvae of Pontopristia amentorum (Foerster) (which live in willow catkins) also have been considered to be organs of locomotion (Conde 1938). Although these swellings are very well developed in Pontopristia larvae, they are as well present in many free-living groups.
All these facts thus support the hypothesis that the concealed feeding habit (either mining or close to mining) was characteristic of Nematinae larvae as long ago as during the earliest stages of their evolution. Among the extant Nematinae, Pseudodineurini (Pseudodineura, Endophytes, and Kerita) are typical leaf miners; an archaic Nearctic species, Caulocampus acericaulis, is a petiole miner. Species of Hoplocampa, the most primitive of the subfamily, bore in fruits of Rosaceae.
Gall-making appears to be a more specialized habit that occurs in advanced Nematinae (Pontania and Euura). Pachynematuspumilio Konow [now Bacconematuspumilio] appears to belong to this ecologic group as well. It inhabits black currant berries inducing abnormal, distorted growth.
A number of Nematinae demonstrate a kind of transitional habit between concealed and free-living ones. Here belong all of Phyllocolpa and also Micronematus monogyniae (Hartig), which produce leaf folds. One may add here Sharliphora ambigua (Fallen), which builds a shelter of young spruce needles connecting them with the excretion of its silk glands, as well as the species of Pontopristia, which live in willow catkins. [Apparently, larvae with transitional habits differ from the free-living ones in their humidity requirements.] Even among the free-feeders, humidity requirements may be quite variable, for example, if one compares those living on upper and lower surface of the leaf blade (Benson 1950).
Some Nematinae species change their habit in the process of larva development. In Pristiphora angulata Lindqvist, the first-instar larvae feed inside Spiraea flower buds, and later instars come out onto the surface of the opening buds or even on the leaves. In this case, the endophytic stage might be non-obligatory.
Among the free-living Amauronematus, there are species that are concealed at the initial stages of development. These are A. viduatus (Zetterstedt) probably together with some other closely related species, which are sometimes segregated into a separate genus Decanematus in accordance with peculiar structure of their ovipositor. The last-instar larvae of A. viduatus have been found feeding free on the leaf surface, whereas their earlier instars usually live concealed inside crowded, distorted upper leaves of young willow shoots. This kind of 'gall', looking much like the damage made by a tortricid, is formed as young leaves lose their ability to grow apart from each other.
There is also evidence that habit changes take place in some Phyllocolpa. The larvae of Ph. purpureae (Cameron) live inside rolled leaves of Salix purpurea and feed on the leaf tissue leaving the upper cuticle intact. On opening an infected leaf at the early stages, one notices an underdeveloped mine with a few excrements and a round hole, through which the larva has exited. All that is left inside the mine is the lower cuticle and a layer of deformed upper parenchyma. When opening leaf rolls at later stages, one no longer can see a conspicuous mine, as the stretched lower cuticle breaks promptly. The upper layer of deformed tissue at the place of oviposition resembles a somewhat underdeveloped Pontania gall and can be recognized even on old leaves.
Phyllocolpa piliserra (Thomson), an inhabitant of rolled leaves of Salix viminalis and S. dasyclados, presents a very different case of habit change. A gregarious feeding habit is typical for the larvae; one may find 6-7 of them together in one leaf roll. Naturally, one leaf cannot provide enough food for all larvae; therefore, at the last instar, they feed freely on the adjacent leaves and use their leaf roll as a shelter during the daytime.
The Phyllocolpa species mentioned above are two extreme examples. Other Phyllocolpa habits may be placed easily between these two. In some species, such as Ph. anglica (Cameron), larvae feed inside the leaf roll during all the instars. In others, like Ph. leucapsis (Tischbein), late instars feed at the leaf margin right near the fold; however, they do not appear to leave their shelter completely while feeding. Yet another way of feeding is seen in Ph. leucosticta (Hartig). Last instars of this species appear to make holes at random on the entire leaf surface (as opposed to Ph. leucapsis and other closely related species that feed at the leaf margins). For that purpose, the larva has to temporarily leave its leaf fold, at least when it is feeding on large leaves of Salixcaprea. These habits culminate with Ph. piliserra mentioned above, which feeds almost freely during its last instar. Species of the closely related genus Nematus that live free during all instars may be considered as a natural extension of this series. The existence of transitional forms may be explained easily if the concealed feeding habit is considered to be primary. It is much more difficult to imagine the opposite way of the evolution.
One of the adaptations of the free-living Nematinae to the water deficit is their cryptonephridial system that has the function of water absorption from the rectum. Only Caulocampus larvae lack the the cryptonephridial system. In this genus, Malpighian tubules are the most primitive. They are loosely positioned, not attached to the intestine (Maxwell 1955). As for all the other species, terminal parts of their Malpighian tubules form a more or less dense, convoluted layer over the surface of the rectum. This facilitates absorbing water from the excrements. Besides, the number of Malpighian tubules has a tendency to increase, which can be easily traced within the subfamily. This fact may be as well attributed to the necessity of saving water in connection with the free-living habit. The smallest number of Malpighian tubes (6-12) is found in Caulocampus,Hoplocampa, Kerita, Pontania, and Euura (Maxwell 1955), all of which have a concealed living habit. In the free-living larvae, the number of Malpighian tubes is as high as 20 to 38 (usually about 28). An intermediate number of Malpighian tubes (14-16) is known only for three species: Priophorus sp., Anoplonyx sp. (Maxwell 1955), and Stauronematus compressicornis (F.) (the latter has about 16, orig. data). These facts as well fit the proposed evolutionary outline of habits in Nematinae. However, they cannot be considered as evidence in favor of the assumption that the concealed feeding habit is the most primitive. One cannot exclude a possibility of a secondary reduction of the Malpighian tubes in case the opposite change from the free to concealed habit has occurred (Maxwell 1955).
As shown by Gilyarov (1949), the concealed habit was ancestral in all the large groups of Holometabola. Ancestors of Symphyta, that is, of all Hymenoptera might have had a more or less concealed habit (Gilyarov 1929, Rasnitsyn 1969). However, problems concerning the evolution of all sawflies are beyond the scope of this article. I would just mention here that the reduction of the antennae is characteristic of not only Nematinae, but also other Tenthredinoidea, such as Diprionidae, Cimbicidae, and Argidae. Among Tenthredinoidea, it is only in the free-living Tenthredinidae and the archaic Blasticotomidae that fully-developed antennae are found. Mining species beside Nematinae are known in Argidae. Some of the free-living Argidae also have pseudocerci.
So far, the free-living habit has been considered to be primary for Tenthredinidae larvae. The presence of prolegs on their abdominal segments has been emphasized in support of this hypothesis. If we accepted that free feeding was primary in all Tenthredinidae and, at the same time, that the concealed habit was primary in Nematinae, then we could conclude that both the free feeding and concealed habit of Nematinae larvae were to be considered secondary within Symphyta. However, the assumption of an evolutuionary inversion, like this, is not sufficiently justified [since we cannot state for sure that the free-living habit was characteristic of the ancestors of Tenthredinidae.]
As a matter of fact, locomotory appendages on the abdominal segments are characteristic of concealed forms as well as free-living ones. In Holometabola larvae, one can trace the development of prolegs and similar appendages many times in different groups. In a number of cases, this development is correlated with the concealed habit rather than free-living habit. One may, therefore, speculate that prolegs had appeared in Symphyta at least earlier than Symphyta completed their transition to the free-living habit and, besides, independently in different groups. In this connection, of particular interest is the fact that many comparatively primitive groups of Tenthredinidae (almost all Dolerini, Selandria of the Selandriini) feed on plants with fistular stems such as horsetails (Equisetum), rushes (Juncus), or grasses. Among the species of Dolerus, there are some that hide inside horsetail stems during the daytime (Lorenz and Kraus 1957).
As for the free-living nematine larvae, not only may we imagine their evolution from some endophytic forms, but also assume that this happened several times, independently, in different groups of the subfamily. Widespread occurrence of the endophytic habit as well as different types of transitional habits among the Nematinae may be considered alone as an indication of this kind of parallel evolution. The development of the cryptonephridial system in Nematinae might have been a prerequisite for their transition to free living, since dehydratation appears to be the most critical factor in connection with the shift toward the free-living habit.
References
Legend
Terminal segments of larvae: 1. Xiphydria sp., dorsal view (after Yuasa 1922); 2. Arthropeas sibirica Loew, dorsal view (after Krivosheina 1968, in part.); 3-4. Nematus (Paranematus) coeruleus Zinovjev, dorsal view; 5. N. (P.) tulunensis Vikberg, last instar; 6-7. Fenusa pusilla (Lepeletier): 6. dorsal view, 7. lateral view; 8-9. Pontania pedunculi (Hartig): 8. dorsal view, 9. lateral view; 10. Phyllocolpa leucaspis (Tischbein), dorsal view; 11. Pamphilius sp., dorsal view.
Antennae of nematine larvae: 12. Nematus hypoxanthus Foerster; 13. Stauronematus compressicornis F.
Pseudocercus in lateral view: 14. Nematus hypoxanthus Foerster
|
|
|
| Salicicola |
Last updated:
webmaster |